Genes su ne ainihin sassan kwayoyin halitta waɗanda ke sarrafa halaye.Sai dai kwayoyin halittar wasu kwayoyin cuta, wadanda suka hada da RNA, kwayoyin halittar mafi yawan halittu sun kunshi DNA..Yawancin cututtuka na kwayoyin halitta suna haifar da mu'amala tsakanin kwayoyin halitta da muhalli.Maganin kwayoyin halitta na iya da gaske warkewa ko rage cututtuka da yawa.Ana ɗaukar magungunan ƙwayoyin halitta a matsayin juyin juya hali a fagen magani da kantin magani.Magungunan maganin kwayoyin halitta a cikin ma'ana mai fa'ida sun haɗa da dogara da magungunan DNA da aka gyara (kamar a cikin vivo magungunan ƙwayoyin cuta dangane da ƙwayoyin cuta, in vitro genetherapy magunguna, tsirara magungunan plasmid, da dai sauransu) da kuma RNA kwayoyi (kamar antisense oligonucleotide kwayoyi, siRNA kwayoyi, da mRNA genetherapy, da dai sauransu);kunkuntar hankali Magungunan jiyya na Halittu galibi sun haɗa da magungunan plasmid DNA, magungunan ƙwayoyin cuta waɗanda suka dogara da ƙwayoyin cuta, magungunan ƙwayoyin cuta waɗanda suka dogara da ƙwayoyin cuta, tsarin gyaran kwayoyin halitta, da magungunan ƙwayoyin cuta don gyare-gyaren halittar in vitro.Bayan shekaru na ci gaba mai raɗaɗi, magungunan ƙwayoyin cuta sun sami sakamako mai ƙarfafawa na asibiti.(Ba a kirga allurar DNA da rigakafin mRNA ba) A halin yanzu, an amince da magungunan jiyya guda 45 don tallatawa a duniya.An amince da jimillar magungunan 9 don tallata su a wannan shekara, gami da hanyoyin jiyya guda 7 da aka amince da su don tallatawa a karon farko a wannan shekara, wato: CARVYKTI, Amvuttra, Upstaza, Roctavian, Hemgenix, Adstiladrin da Ebvallo, (Lura: Sauran biyun an amince da su a Amurka a wannan shekarar. don tallace-tallace a Amurka a watan Agusta 2022, kuma Tarayyar Turai ta amince da ita don tallata a cikin 2019; .) Tare da ƙaddamar da ƙarin samfuran maganin kwayoyin halitta da saurin haɓaka fasahar maganin kwayoyin halitta, maganin ƙwayoyin cuta na gab da kawo ƙarshen ci gaba cikin sauri.
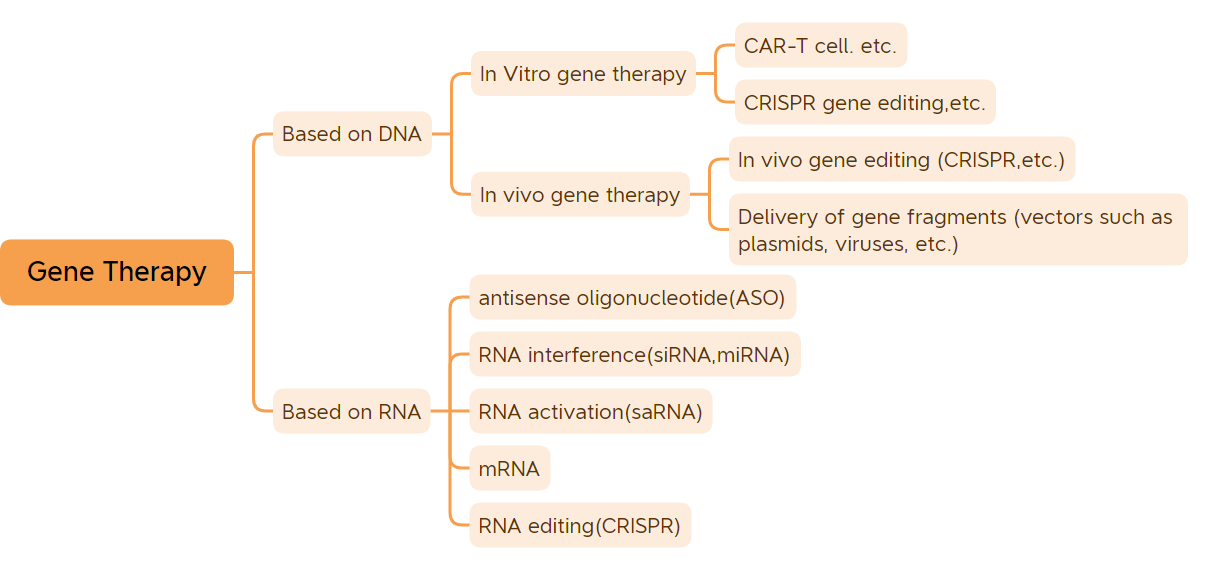
Rabe-raben jiyya na kwayoyin halitta (Tsarin Hoto: Bio-Matrix)
Wannan labarin ya jera hanyoyin jiyya guda 45 (ban da allurar DNA da rigakafin mRNA) waɗanda aka amince da su don talla.
1. In vitro genetherapy
(1) Strimvelis
Kamfanin: GlaxoSmithKline (GSK) ne ya haɓaka.
Lokacin zuwa kasuwa: Tarayyar Turai ta amince da ita don talla a watan Mayu 2016.
Alamomi: Don maganin matsanancin rashin ƙarfi na rigakafi (SCID).
Bayani: Babban tsarin wannan farfaganda shine fara samun nasu kwayoyin halittar hematopoietic na majiyyaci, fadadawa da al'ada su a cikin vitro, sannan a yi amfani da retrovirus don gabatar da kwafin ADA (adenosine deaminase) mai aiki a cikin sel na hematopoietic, kuma a karshe allurar da aka gyara Hematopoietic stem cell an sake dawo da su cikin jiki.Sakamakon asibiti ya nuna cewa shekarun rayuwa na shekaru 3 na marasa lafiya ADA-SCID da aka yi wa Strimvelis shine 100%.
(2) Zalmoxis
Kamfanin: Kamfanin Italiya MolMed ne ya samar.
Lokacin zuwa kasuwa: Samu izinin tallan sharadi daga Tarayyar Turai a cikin 2016.
Alamomi: Ana amfani da shi don maganin adjuvant na tsarin rigakafi na marasa lafiya bayan dashen kwayar cutar hematopoietic.
Bayani: Zalmoxis wani allogeneic T cell kashe kansa gene immunotherapy gyara ta retroviral vectors.Wannan hanya tana amfani da vectors retroviral don canza kwayoyin halitta ta allogeneic T Kwayoyin, don haka kwayoyin halittar T da aka canza su bayyana 1NGFR da HSV-TK Mut2 kwayoyin kashe kansa suna ba da damar mutane suyi amfani da magungunan ganciclovir (ganciclovir) a kowane lokaci don kashe kwayoyin T wanda ke haifar da mummunan halayen rigakafi, hana yiwuwar kara lalacewa na GVHD, da kuma samar da aikin rigakafi na baya-bayan nan na GVHD.
(3) Invossa-K
Kamfanin: TissueGene (KolonTissueGene) ya haɓaka.
Lokacin kasuwa: An amince da jeri a Koriya ta Kudu a cikin Yuli 2017.
Alamomi: Don maganin cututtukan ciwon gwiwa na degenerative.
Bayanan Bayani: Invossa-K shine maganin kwayoyin halitta na allogeneic wanda ya shafi chondrocytes na mutum.Kwayoyin allogeneic an gyare-gyare ta hanyar kwayoyin halitta a cikin vitro, kuma sel da aka gyara zasu iya bayyanawa da ɓoye abubuwan haɓaka girma β1 (TGF-β1) bayan allurar intra-articular.β1), don haka inganta alamun osteoarthritis.Sakamakon asibiti ya nuna cewa Invossa-K na iya inganta ciwon gwiwa sosai.Hukumar Abinci da Magunguna ta Koriya ta soke ta a cikin 2019 saboda masana'anta sun bata sunayen sinadaran da aka yi amfani da su.
(4) Zynteglo
Kamfanin: Bincike da haɓaka ta bluebird bio.
Lokacin kasuwa: Tarayyar Turai ta amince da tallata a cikin 2019, kuma FDA ta amince da ita don tallata a Amurka a watan Agusta 2022.
Alamomi: Don maganin β-thalassemia mai dogaro da jini.
Bayani: Zynteglo shine maganin lentiviral in vitro wanda ke gabatar da kwafin aiki na kwayar halittar β-globin ta al'ada (βA-T87Q-globin gene) zuwa cikin kwayoyin halitta na hematopoietic da aka dauka daga majiyyaci ta hanyar kwayar cutar lentiviral, sannan kuma ta sake dawo da wadannan kwayoyin halittar da aka gyara autologous hematopoietic stem cell cikin majiyyaci.Da zarar majiyyaci yana da kwayar halittar βA-T87Q-globin ta al'ada, za su iya samar da furotin na HbAT87Q na yau da kullun, wanda zai iya ragewa ko kawar da buƙatar ƙarin jini yadda ya kamata.Jiyya ce ta lokaci ɗaya da aka ƙera don maye gurbin ƙarin ƙarin jini na rayuwa da magunguna na tsawon rai ga marasa lafiya masu shekaru 12 zuwa sama.
(5) Skysona
Kamfanin: Bincike da haɓaka ta bluebird bio.
Lokaci don kasuwa: Tarayyar Turai ta amince da siyarwa a cikin Yuli 2021, kuma FDA ta amince da ita don tallata a Amurka a cikin Satumba 2022.
Alamomi: Don maganin farkon cerebral adrenoleukodystrophy (CALD).
Bayani: Maganin kwayoyin halittar Skysona shine kawai maganin kwayoyin halitta na lokaci daya da aka amince da shi don maganin adrenoleukodystrophy na cerebral na farko (CALD).Skysona (elivaldogene autotemcel, Lenti-D) wani hematopoietic stem cell lentiviral in vitro gene far Lenti-D.Gabaɗaya tsarin jiyya shine kamar haka: ana fitar da ƙwayoyin hematopoietic masu ƙarfi na autologous daga mai haƙuri, an canza su kuma an canza su ta hanyar lentivirus ɗauke da kwayar halittar ABCD1 na ɗan adam a cikin vitro, sannan a mayar da su ga mai haƙuri.Ana amfani dashi don kula da marasa lafiya a ƙarƙashin shekarun 18, ɗauke da maye gurbi na ABCD1, da CALD.
(6) Kymriah
Kamfanin: Novartis ne ya haɓaka.
Lokacin zuwa kasuwa: FDA ta amince da tallan a watan Agusta 2017.
Alamomi: Jiyya na precursor B-cell m lymphoblastic cutar sankarar bargo (ALL) da koma baya da kuma refractory DLBCL.
Jawabai: Kymriah magani ne na lentiviral in vitro genetherapy, farkon CAR-T far da aka amince don tallatawa a duniya, yana nufin CD19, da kuma amfani da 4-1BB co-stimulatory factor.Ana siyar da shi a $475,000 a Amurka da $313,000 a Japan.
(7) Yaskarta
Kamfanin: Kite Pharma, reshen Gileyad (GILD) ne ya haɓaka.
Lokaci zuwa kasuwa: FDA ta amince da tallace-tallace a watan Oktoba 2017;Fosun Kite ta gabatar da fasahar Yescarta daga Kite Pharma kuma ta kera ta a kasar Sin bayan samun izini.An amince da jeri a cikin ƙasar.
Alamomi: Don maganin sake dawowa ko refractory babban lymphoma B-cell.
Jawabai: Yescarta magani ne na sake dawowa a cikin vitro, wanda shine na biyu da aka yarda da CAR-T a duniya.Yana kai hari CD19 kuma yana ɗaukar mai costimulator na CD28.Ana siyar dashi akan $373,000 a Amurka.
(8) Takardu
Kamfanin: Gileyad (GILD) ne ya haɓaka.
Lokacin zuwa kasuwa: FDA ta amince da tallata a watan Yuli 2020.
Alamomi: Don sake dawowa ko refractory mantle cell lymphoma.
Bayani: Tecartus magani ne mai sarrafa kansa na CAR-T wanda ke niyya CD19, kuma shine magani na CAR-T na uku da aka amince don tallatawa a duniya.
(9) Breyanzi
Kamfanin: Bristol-Myers Squibb (BMS) ya haɓaka.
Lokacin zuwa kasuwa: FDA ta amince da ita don talla a cikin Fabrairu 2021.
Alamomi: Relapsed ko refractory (R/R) babban B-cell lymphoma (LBCL).
Jawabai: Breyanzi magani ne na in vitro wanda ya dogara da lentivirus, magani na CAR-T na huɗu da aka amince da shi don tallatawa a duniya, yana nufin CD19.Yarda da Breyanzi wani ci gaba ne ga Bristol-Myers Squibb a fagen rigakafin rigakafi ta salula, wanda ya samu lokacin da ya sami Celgene akan dala biliyan 74 a cikin 2019.
(10)Abba
Kamfanin: Haɗin gwiwar Bristol-Myers Squibb (BMS) da bluebird bio.
Lokacin kasuwa: FDA ta amince da ita don talla a cikin Maris 2021.
Alamomi: sake dawowa ko refractory mahara myeloma.
Jawabai: Abecma magani ne na in vitro wanda ya dogara da lentivirus, maganin tantanin halitta na CAR-T na farko a duniya wanda ke nufin BCMA, da kuma magani na CAR-T na biyar da FDA ta amince.Ka'idar miyagun ƙwayoyi ita ce bayyana masu karɓa na BCMA na chimeric akan ƙwayoyin T na majiyyaci ta hanyar gyare-gyaren ƙwayoyin cuta na lentivirus a cikin vitro.Jiyya don kawar da ƙwayoyin T waɗanda ba a canza su ba a cikin marasa lafiya, sa'an nan kuma sake dawo da ƙwayoyin T da aka gyara, waɗanda ke neman kuma suna kashe BCMA-bayyana ƙwayoyin ciwon daji a cikin marasa lafiya.
(11) Libmeldy
KAMFANI: Orchard Therapeutics ne ya haɓaka.
Lokacin kasuwa: Tarayyar Turai ta amince da yin lissafin a watan Disamba 2020.
Alamomi: Don maganin metachromatic leukodystrophy (MLD).
Bayani: Libmeldy magani ne na kwayoyin halitta wanda ya dogara da CD34+ sel masu zaman kansu waɗanda aka gyara su cikin vitro ta hanyar lentivirus.Bayanai na asibiti sun nuna cewa jiko guda ɗaya na Libmeldy na iya canza yanayin farkon MLD yadda ya kamata idan aka kwatanta da matsananciyar motsi da nakasar fahimi a cikin marasa lafiya da ba a kula da su ba na shekaru ɗaya.
(12) Benoda
Kamfanin: WuXi Giant Nuo ne ya haɓaka.
Lokacin kasuwa: NMPA ta amince da shi bisa hukuma a cikin Satumba 2021.
Alamomi: Jiyya na tsofaffi marasa lafiya tare da relapsed ko refractory babban B-cell lymphoma (r / r LBCL) bayan layi na biyu ko sama da tsarin tsarin.
Bayani: Beinoda maganin kwayoyin halittar CD19 CAR-T ne, kuma shine ainihin samfurin Kamfanin WuXi Juro.Wannan shi ne samfurin mota na biyu a kasar Sin, ban da jan hankali / gyaran tantanin halitta (cll), ya diceemi, na biyu. mymphobollastic leuckedia (duka).
(13) CARVYKTI
Kamfanin: Samfurin farko na Legend Biotech wanda aka amince da shi don talla.
Lokacin zuwa kasuwa: FDA ta amince da ita don talla a cikin Fabrairu 2022.
Alamomi: don maganin sake dawowa ko refractory mahara myeloma (R / R MM).
Jawabai: CARVYKTI (ciltacabtagene autoleucel, Cilta-cel a takaice) maganin rigakafi ne na kwayar halitta ta CAR-T tare da ƙwayoyin rigakafi guda biyu waɗanda ke nufin antigen maturation B-cell (BCMA).Bayanai sun nuna cewa CARVYKTI A cikin marasa lafiya tare da relapsed ko refractory mahara myeloma wadanda suka karbi hudu ko fiye kafin hanyoyin kwantar da hankali (ciki har da proteasome inhibitors, immunomodulators da anti-CD38 monoclonal antibodies), wani jimlar amsa yawan 98% an nuna.
(14)Ebvallo
Kamfanin: Atara Biotherapeutics ne ya haɓaka.
Hukumar Tarayyar Turai (EC) don tallace-tallace a cikin Disamba 2022, ita ce farkon duniya ta T cell far da aka amince don tallatawa.
Alamomi: A matsayin monotherapy don cutar Epstein-Barr (EBV) da ke da alaƙa bayan dasawa da cutar lymphoproliferative (EBV + PTLD), marasa lafiya da ke karɓar magani dole ne su zama manya da yara sama da shekaru 2 waɗanda a baya sun karɓi aƙalla wani magani na magani.
Bayanan Bayani: Ebvallo shine allogeneic EBV-takamaiman maganin kwayoyin halittar T-cell na duniya wanda ke hari da kuma kawar da kwayoyin cutar EBV a cikin takurawa HLA.Yarda da wannan maganin ya dogara ne akan sakamakon binciken gwaji na asibiti na 3 mai mahimmanci, kuma sakamakon ya nuna cewa ORR na ƙungiyar HCT da ƙungiyar SOT shine 50%.Matsakaicin adadin gafara (CR) shine 26.3%, ƙimar juzu'i (PR) shine 23.7%, kuma matsakaicin lokacin gafara (TTR) shine watanni 1.1.Daga cikin marasa lafiya 19 da suka sami gafara, 11 suna da tsawon lokacin amsawa (DOR) fiye da watanni 6.Bugu da ƙari, dangane da aminci, babu wani mummunan halayen da ya faru kamar cutar da ake kira graft-versus-host disease (GvHD) ko ciwon sakin cytokine mai alaka da Ebvallo.
2. In vivo genetherapy dangane da ƙwayoyin cuta
(1) Gendicine/Jin Sheng
Kamfanin: Kamfanin Shenzhen Saibainuo ne ya haɓaka.
Lokacin kasuwa: An yarda da za a jera su a China a cikin 2003.
Alamomi: Don maganin ciwon kai da wuyansa squamous cell carcinoma.
Lura: Recombinant ɗan adam p53 allurar adenovirus Gendicine/Jinyousheng magani ne na adenovirus vector therapy tare da haƙƙin mallakar fasaha mai zaman kansa mallakar Kamfanin Shenzhen Saibainuo.Nau'in mutum na 5 adenovirus ya ƙunshi nau'in adenovirus na ɗan adam 5. Na farko shine babban tsari don tasirin maganin ƙwayar cuta, kuma na ƙarshe yana aiki a matsayin mai ɗauka.Kwayar cuta ta adenovirus tana ɗaukar kwayar cutar p53 a cikin kwayar da aka yi niyya, yana bayyana kwayar cutar ciwon daji p53 a cikin tantanin halitta da aka yi niyya, da kuma bayanin halittarsa Samfurin zai iya daidaita nau'ikan kwayoyin cutar kansa da rage-kayyade ayyukan nau'ikan oncogenes iri-iri, ta haka yana haɓaka ciwace-ciwacen jiki da ke hana ciwace-ciwacen dalili da kashe ciwace-ciwace.
(2) Rikicin
Kamfanin: Kamfanin Latima ne ya haɓaka, Latvia.
Lokacin jeri: An amince da jeri a Latvia a cikin 2004.
Alamomi: Don maganin melanoma.
Bayani: Rigvir magani ne na kwayoyin halitta wanda ya danganci vector ECHO-7 da aka gyara ta hanyar kwayoyin halitta.A halin yanzu, an karɓi maganin a Latvia, Estonia, Poland, Armenia, Belarus, da sauransu, kuma ana yin rajistar EMA a cikin ƙasashen EU.Abubuwan da suka shafi asibiti a cikin shekaru goma da suka gabata sun tabbatar da cewa kwayar cutar oncolytic Rigvir tana da lafiya kuma tana da inganci, kuma tana iya ƙara yawan rayuwar marasa lafiyar melanoma da sau 4-6.Bugu da kari, wannan farfagandar kuma ana amfani da ita ga wasu nau'ikan cututtukan daji, wadanda suka hada da kansar launin fata, kansar pancreatic, kansar mafitsara Ciwon daji, kansar koda, kansar prostate, kansar huhu, kansar mahaifa, lymphosarcoma, da sauransu.
(3) Kankara
Kamfanin: Kamfanin Shanghai Sanwei Biological Company ne ya haɓaka.
Lokacin kasuwa: An yarda da za a jera su a China a cikin 2005.
Alamomi: maganin ciwon kai da wuyansa, ciwon hanta, kansar pancreatic, kansar mahaifa da sauran cututtukan daji.
Bayani: Oncorine (安科瑞) samfurin maganin ƙwayoyin cuta ne na oncolytic ta amfani da adenovirus a matsayin mai ɗauka.Ana samun adenovirus oncolytic, wanda zai iya yin kwafi na musamman a cikin p53 rashi ko ciwace-ciwacen ciwace-ciwace, wanda ke haifar da lysis na ƙwayoyin tumor, ta haka ne ke kashe ƙwayoyin tumor.ba tare da lalata ƙwayoyin al'ada ba.Nazarin asibiti ya nuna cewa Ankerui yana da lafiya mai kyau da inganci ga nau'ikan ciwace-ciwacen daji iri-iri.
(4) Glybera
Kamfanin: UniQure ne ya haɓaka.
Lokacin kasuwa: An amince da jeri a Turai a cikin 2012.
Alamomi: Maganin rashi lipoprotein lipase (LPLD) tare da mai tsanani ko maimaita lokuta na pancreatitis duk da ƙuntataccen abincin mai mai.
Bayani: Glybera (alipogene tiparvovec) magani ne na maganin kwayoyin halitta wanda ya dogara da AAV, wanda ke amfani da AAV a matsayin mai ɗaukar hoto don canza kwayar halitta ta LPL a cikin ƙwayoyin tsoka, don haka kwayoyin da suka dace zasu iya samar da wani nau'i na lipoprotein lipase, Don kawar da cutar, wannan farfadowa yana da tasiri na dogon lokaci (magunguna).An cire miyagun ƙwayoyi daga kasuwa a cikin 2017. Dalilin janyewar na iya kasancewa da alaka da abubuwa biyu: babban farashi da ƙayyadaddun buƙatun kasuwa.Matsakaicin kudin magani na maganin ya kai dalar Amurka miliyan 1, kuma majiyyaci daya ne kawai ya saya ya yi amfani da shi ya zuwa yanzu.Ko da yake kamfanin inshorar likitanci ya biya dalar Amurka 900,000 a gare shi, amma kuma nauyi ne mai yawa ga kamfanin inshora.Bugu da kari, alamun da aka yi niyya da maganin ba su da yawa, tare da adadin abubuwan da suka faru kusan 1 cikin miliyan 1 da kuma yawan rashin ganewa.
(5) Ilygic
Kamfanin: Amgen ne ya haɓaka.
Lokacin kasuwa: A cikin 2015, an amince da shi don a jera shi a cikin Amurka da Tarayyar Turai.
Alamomi: Maganin ciwon melanoma waɗanda ba za a iya cire su gaba ɗaya ta hanyar tiyata ba.
Bayani: Imlygic shine nau'in kwayar cutar herpes simplex da aka rage ta nau'in 1 wanda aka gyara ta hanyar fasahar kwayoyin halitta (share ICP34.5 da ICP47 gutsuttsuran kwayoyin halitta, da shigar da kwayar halittar granulocyte macrophage colony-stimulating factor GM-CSF a cikin kwayar cutar) (HSV-1) kwayar cutar oncolytic shine farkon kwayar cutar ta FDA-approv.Hanyar gudanarwa ita ce allurar intralesional, wanda za a iya yin allurar kai tsaye a cikin raunuka na melanoma don haifar da fashewar ƙwayoyin tumor, saki antigens da aka samo daga tumor da GM-CSF, da kuma inganta amsawar rigakafin ciwon daji.
(6) Luxturna
Kamfanin: Spark Therapeutics, wani reshen Roche ne ya haɓaka.
Lokaci don kasuwa: FDA ta amince da ita don tallace-tallace a cikin 2017, sannan kuma an amince da ita don tallace-tallace a Turai a cikin 2018.
Alamomi: Don kula da yara da manya waɗanda suka yi hasarar gani saboda kwafi biyu na RPE65 maye gurbi amma suna riƙe isassun adadin ƙwayoyin retinal masu dacewa.
Jawabai: Luxturna magani ne na tushen AAV wanda aka yi ta allurar subretina.Maganin kwayoyin halitta yana amfani da AAV2 a matsayin mai ɗaukar hoto don gabatar da kwafin aiki na al'ada RPE65 na al'ada a cikin sel na retinal na marasa lafiya, don haka sel masu dacewa suna bayyana furotin RPE65 na al'ada, suna samar da ƙarancin furotin RPE65 na mai haƙuri, don haka inganta hangen nesa mai haƙuri.
(7) Zolgensma
Kamfanin: AveXis, wani reshen Novartis ne ya haɓaka.
Lokacin zuwa kasuwa: FDA ta amince da ita don talla a watan Mayu 2019.
Alamomi: Jiyya na Atrophy Muscular Atrophy (SMA) marasa lafiya a ƙarƙashin shekaru 2.
Bayani: Zolgensma magani ne na kwayoyin halitta wanda ya danganci vector AAV.Wannan magani shine kawai tsarin jiyya na lokaci ɗaya don atrophy na muscular na kashin baya da aka amince don tallatawa a duniya.Kaddamar da miyagun ƙwayoyi yana buɗe sabon zamani a cikin jiyya na kashin baya na muscular atrophy.shafi, babban ci gaba ne.Wannan maganin kwayoyin halitta yana amfani da vector na scAAV9 don gabatar da al'ada na SMN1 na al'ada a cikin mai haƙuri ta hanyar jiko don samar da furotin na SMN1 na al'ada, don haka inganta aikin ƙwayoyin da aka shafa kamar su neurons.Sabanin haka, magungunan SMA Spinraza da Evrysdi suna buƙatar maimaita allurai na dogon lokaci.Ana ba da Spinraza ta hanyar allurar kashin baya kowane wata hudu, kuma Evrysdi magani ne na baka na yau da kullun.
(8) Tsarkakewa
Kamfanin: Daiichi Sankyo Company Limited ne ya haɓaka (TYO: 4568).
Lokaci don kasuwa: Yarda da sharadi daga Ma'aikatar Lafiya, Ma'aikata da Jin Dadin Jama'a (MHLW) a cikin Yuni 2021.
Alamomi: Don maganin cutar glioma.
Bayani: Delytact shine samfur na huɗu na maganin ƙwayoyin cuta na oncolytic da aka amince da shi a duniya, kuma samfurin ƙwayar cuta na oncolytic na farko da aka amince da shi don maganin glioma.Delytact wata cuta ce da aka yi amfani da ita ta hanyar kwayoyin cutar ta herpes simplex irin 1 (HSV-1) cutar oncolytic da Dr. Todo da abokan aiki suka kirkira.Delytact yana gabatar da ƙarin maye gurbi a cikin G207 genome na ƙarni na biyu na HSV-1, yana haɓaka zaɓinsa na zaɓi a cikin ƙwayoyin cutar kansa da shigar da martanin rigakafin ƙwayar cuta yayin kiyaye babban aminci.Delytact shine ƙarni na uku na oncolytic HSV-1 a halin yanzu yana jurewa gwajin asibiti.Amincewar Delytact a Japan ya dogara ne akan gwaji na asibiti lokaci na hannu guda 2.A cikin marasa lafiya tare da glioblastoma na yau da kullun, Delytact ya sami ƙarshen ƙarshen ƙarshen rayuwa na shekara guda, kuma sakamakon ya nuna cewa Delytact ya nuna ingantaccen inganci idan aka kwatanta da G207.Ƙarfin kwafi mai ƙarfi da aikin antitumor mafi girma.Wannan yana da tasiri a cikin ƙayyadaddun nau'in ciwon daji na nono, prostate, schwannomas, nasopharyngeal, hepatocellular, colorectal, m na gefen jijiya ciwon ciwace-ciwacen daji, da ciwon daji na thyroid.
(9) Tafiya
Kamfanin: PTC Therapeutics, Inc. ya haɓaka (NASDAQ: PTCT).
Lokacin kasuwa: Tarayyar Turai ta amince da tallan a watan Yuli 2022.
Alamomi: Don rashi L-amino acid decarboxylase (AADC), an yarda da shi don kula da marasa lafiya masu shekaru 18 da haihuwa.
Bayani: Upstaza™ (eladocagene exuparvovec) magani ne a cikin vivo tare da nau'in ƙwayar cuta mai alaƙa da adeno 2 (AAV2) azaman mai ɗaukar hoto.Marasa lafiya sun yi rashin lafiya saboda maye gurbi a cikin kwayar halittar da ke ɓoye enzyme AADC.AAV2 yana ɗaukar kwayar halitta mai lafiya wanda ke ɓoye enzyme AADC.Siffar ramuwa ta kwayoyin halitta tana samun sakamako na warkewa.A ka'idar, gudanarwa ɗaya yana da tasiri na dogon lokaci.Ita ce farkon da aka fara sayar da maganin kwayoyin halitta wanda ake allura kai tsaye a cikin kwakwalwa.Izinin tallan ya shafi duk ƙasashe membobin EU 27, da Iceland, Norway da Liechtenstein.
(10) Roctavian
Kamfanin: BioMarin Pharmaceutical (BioMarin) ya haɓaka.
Lokacin kasuwa: Tarayyar Turai ta amince da talla a watan Agusta 2022;izinin tallace-tallace ta Hukumar Kula da Magunguna da Kula da Lafiya ta Burtaniya (MHRA) a cikin Nuwamba 2022.
Alamomi: Don kula da tsofaffi marasa lafiya tare da hemophilia mai tsanani A waɗanda ba su da tarihin hana FVIII factor kuma ba su da kyau ga kwayoyin AAV5.
Bayani: Roctavian (valoctocogene roxaparvovec) yana amfani da AAV5 a matsayin vector kuma yana amfani da HLP na musamman mai haɓaka hanta don fitar da bayyanar cututtukan coagulation na mutum VIII (FVIII) tare da share yankin B.Matakin da Hukumar Tarayyar Turai ta yanke na amincewa da tallan valoctocogene roxaparvovec ya dogara ne akan jimillar bayanan aikin ci gaban asibiti na miyagun ƙwayoyi.Daga cikin su, sakamakon gwajin gwaji na gwaji na III na asibiti GENER8-1 ya nuna cewa idan aka kwatanta da bayanan shekarar kafin yin rajista, bayan jiko ɗaya na valoctocogene roxaparvovec, Matsalolin jini na shekara-shekara (ABR) yana raguwa sosai, yawan amfani da shirye-shiryen furotin na recombinant coagulation VIII (F8) an rage shi sosai, ko kuma ƙara yawan aikin jini a cikin F8.Bayan makonni 4 na jiyya, ƙimar amfani da F8 na shekara-shekara da ABR na buƙatar magani an rage su da 99% da 84%, bi da bi, kuma bambancin yana da mahimmanci a ƙididdiga (p<0.001).Bayanan martaba na aminci yana da kyau, kuma babu wani batu da ya fuskanci hanawar F8 factor, malignancy ko thrombosis sakamako masu illa, kuma ba a ba da rahoton wani mummunan al'amuran da suka shafi jiyya (SAEs).
(11) Hemgenix
Kamfanin: UniQure Corporation ne ya haɓaka.
Lokacin zuwa kasuwa: FDA ta amince da ita don talla a watan Nuwamba 2022.
Alamomi: don kula da manya marasa lafiya tare da hemophilia B.
Bayani: Hemgenix magani ne na kwayoyin halitta wanda ya danganci vector AAV5.An sanye da magungunan tare da nau'in nau'in coagulation factor IX (FIX) bambance-bambancen gene FIX-Padua, wanda ake gudanarwa ta cikin jini.Bayan gudanarwa, kwayar halitta na iya bayyana FIX coagulation factor a cikin hanta kuma ya ɓoye Bayan shigar da jini don yin aikin coagulation, don cimma manufar jiyya, a ka'idar, gudanarwa ɗaya yana da tasiri na dogon lokaci.
(12) Adstiladrin
Kamfanin: Ferring Pharmaceuticals ne ya haɓaka .
Lokacin zuwa kasuwa: FDA ta amince da ita don talla a cikin Disamba 2022.
Alamomi: Don maganin ciwon daji mai haɗari mai haɗari wanda ba na tsoka ba (NMIBC) ba ya amsa ga Bacillus Calmette-Guerin (BCG) .
Bayani: Adstiladrin magani ne na kwayoyin halitta wanda ya dogara ne akan wani nau'i na adenoviral wanda ba ya maimaitawa, wanda zai iya yin overexpress interferon alfa-2b protein a cikin kwayoyin da aka yi niyya, kuma ana gudanar da shi ta hanyar catheter na fitsari a cikin mafitsara (ana gudanar da shi sau ɗaya a kowane watanni uku) , ƙwayar ƙwayar cuta na iya kamuwa da kwayar cutar ta yadda ya kamata a cikin sel na bangon mafitsara, sa'an nan kuma sunadarin da ke haifar da interferon.Wannan sabuwar hanyar maganin kwayoyin halitta don haka tana canza ƙwayoyin bangon mafitsara na majiyyaci zuwa ƙaramin “masana’anta” wanda ke samar da interferon, ta haka yana haɓaka ƙarfin majiyyaci na yaƙi da cutar kansa.
An kimanta aminci da ingancin Adstiladrin a cikin binciken asibiti da yawa ciki har da marasa lafiya 157 tare da babban haɗarin BCG-marasa amsa NMIBC.Marasa lafiya sun karɓi Adstiladrin kowane wata uku har zuwa watanni 12, ko kuma har sai daɗaɗɗen da ba za a yarda da su ba ga jiyya ko maimaita babban darajar NMIBC.Gabaɗaya, kashi 51 cikin 100 na marasa lafiya da aka yiwa rajista tare da Adstiladrin sun sami cikakkiyar amsa (bacewar duk alamun ciwon daji da aka gani akan cystoscopy, ƙwayar biopsy, da fitsari).
3. Kananan magungunan nucleic acid
(1) Vitravene
Kamfanin: Haɗin gwiwa ta hanyar Ionis Pharma (tsohon Isis Pharma) da Novartis.
Lokacin kasuwa: A cikin 1998 da 1999, FDA da EU EMA sun amince da ita don talla.
Alamomi: Don maganin cytomegalovirus retinitis a cikin marasa lafiya masu cutar HIV.
Bayani: Vitravene magani ne na oligonucleotide na antisense, wanda shine maganin oligonucleotide na farko da aka amince da shi don kasuwanci a duniya.A matakin farko na jeri, buƙatun kasuwa na magungunan rigakafin CMV ya kasance cikin gaggawa;daga baya, saboda ci gaban da ake samu sosai na maganin cutar kanjamau, adadin cututtukan CMV ya ragu sosai.Saboda jajircewar kasuwa, an ƙaddamar da maganin a cikin 2002 da 2006 janyewar a cikin ƙasashen EU da Amurka.
(2)Magu
Kamfanin: Pfizer da Eyetech ne suka haɓaka.
Lokacin kasuwa: An amince da jeri a Amurka a cikin 2004.
Alamomi: Don maganin cututtukan da ke da alaƙa da macular degeneration shekaru neovascular.
Bayani: Macugen magani ne na oligonucleotide wanda aka gyara pegylated, wanda zai iya yin niyya da kuma ɗaure ma'aunin haɓakar haɓakar jijiyoyi (VEGF165 subtype), kuma hanyar gudanarwa shine allurar intravitreal.
(3) Defitelio
Kamfanin: Jazz Pharmaceuticals ne ya haɓaka.
Lokacin zuwa kasuwa: Ƙungiyar Tarayyar Turai ta amince da ita don tallace-tallace a cikin 2013 kuma FDA ta amince da ita don tallace-tallace a cikin Maris 2016.
Alamomi: Don maganin cututtukan hanta da ke hade da rashin aikin koda ko na huhu bayan dashen kwayar cutar hematopoietic.
Bayani: Defitelio magani ne na oligonucleotide, wanda shine cakuda oligonucleotides tare da abubuwan plasmin.Janye daga kasuwa a cikin 2009 saboda dalilai na kasuwanci.
(4) Kynamro
Kamfanin: Ionis Pharma da Kastle ne suka haɓaka.
Lokacin kasuwa: A cikin 2013, an amince da ita don tallatawa a Amurka azaman maganin marayu.
Alamomi: Don maganin adjuvant na homozygous familial hypercholesterolemia.
Bayani: Kynamro magani ne na oligonucleotide antisense, wanda shine maganin antisense oligonucleotide wanda ke nufin apo B-100 mRNA na ɗan adam.Ana gudanar da Kynamro a matsayin 200 MG subcutaneously sau ɗaya a mako.
(5) Spinraza
Kamfanin: Ionis Pharmaceuticals ne ya haɓaka.
Lokaci don kasuwa: FDA ta amince da ita don tallace-tallace a watan Disamba 2016.
Alamomi: Don maganin atrophy na muscular na kashin baya (SMA).
Bayani: Spinraza (nusinersen) maganin antisense oligonucleotide ne.Ta hanyar ɗaure zuwa wurin tsagewar SMN2 exon 7, Spinraza na iya canza ɓangarorin RNA na kwayar halittar SMN2, ta haka ƙara samar da furotin SMN mai cikakken aiki.A cikin watan Agusta 2016, BIOGEN ta yi amfani da zaɓin ta don samun haƙƙin duniya na Spinraza.Spinraza kawai ya fara gwaji na asibiti na farko a cikin mutane a cikin 2011. A cikin shekaru 5 kawai, FDA ta amince da ita don tallace-tallace a 2016, wanda ke nuna cikakkiyar amincewar FDA game da ingancinta.An amince da maganin don yin tallace-tallace a kasar Sin a watan Afrilun 2019. Dukkanin sake zagayowar amincewa ga Spinraza a kasar Sin bai wuce watanni 6 ba, kuma shekaru 2 da watanni 2 kenan tun lokacin da aka fara amincewa da Spinraza a Amurka.Gudun jeri a China ya riga ya yi sauri sosai.Wannan kuma ya faru ne saboda gaskiyar cewa Cibiyar Nazarin Magunguna ta ba da "Sanarwa akan Buga Lissafin Farko na Sabbin Magungunan Ketare da ake Bukatar gaggawa a Ayyukan Clinical" a ranar 1 ga Nuwamba, 2018, kuma an haɗa shi a cikin rukunin farko na 40 sababbin magunguna na kasashen waje don sake dubawa, wanda Spinraza ya kasance cikin matsayi.
(6) Fitowa ta 51
Kamfanin: AVI BioPharma ne ya haɓaka (daga baya aka sake masa suna Sarepta Therapeutics).
Lokacin zuwa kasuwa: A watan Satumba na 2016, FDA ta amince da ita don tallace-tallace.
Alamomi: Don maganin Duchenne muscular dystrophy (DMD) tare da exon 51 skipping gene maye gurbi a cikin DMD gene.
Bayani: Exondys 51 maganin antisense oligonucleotide ne, maganin antisense oligonucleotide na iya ɗaure zuwa matsayin exon 51 na pre-mRNA na DMD gene, wanda ya haifar da samuwar mRNA balagagge, wani ɓangare na exon 51 an toshe Excision, ta haka ne wani ɓangare na gyara tsarin karatun mRNA na yau da kullun, yana taimakawa dystrophin na yau da kullun don inganta tsarin karatun mRNA. alamomin mara lafiya.
(7) Tegsedi
Kamfanin: Ionis Pharmaceuticals ne ya haɓaka.
Lokacin zuwa kasuwa: Tarayyar Turai ta amince da ita don talla a cikin Yuli 2018.
Alamomi: don maganin gadon gado na transthyretin amyloidosis (hATTR).
Bayani: Tegsedi maganin antisense oligonucleotide ne wanda ke nufin transthyretin mRNA.Ita ce magani na farko da aka amince da shi a duniya don maganin hatTR.Ana gudanar da shi ta hanyar allurar subcutaneous.Magungunan yana rage samar da furotin na ATTR ta hanyar yin niyya ga mRNA na transthyretin (ATTR), kuma yana da rabo mai fa'ida mai kyau a cikin jiyya na ATTR, kuma an inganta yanayin neuropathy da ingancin rayuwa na mai haƙuri, kuma ya dace da nau'ikan maye gurbin TTR, Babu matakin cuta ko kasancewar cardiomyopathy ya dace.
(8) Tafiya
Kamfanin: Kamfanin Alnylam Corporation da Kamfanin Sanofi ne suka haɓaka.
Lokacin kasuwa: An amince da jeri a cikin Amurka a cikin 2018.
Alamomi: don maganin gadon gado na transthyretin amyloidosis (hATTR).
Bayani: Onpattro shine siRNA magani wanda ke nufin transthyretin mRNA, wanda ke rage samar da furotin ATTR a cikin hanta kuma yana rage tarin amyloid adibas a cikin jijiyoyi na gefe ta hanyar niyya mRNA na transthyretin (ATTR), don haka ingantawa da rage alamun cututtuka.
(9) Givlaari
Kamfanin: Alnylam Corporation ne ya haɓaka.
Lokacin zuwa kasuwa: FDA ta amince da ita don talla a watan Nuwamba 2019.
Alamomi: Don maganin m hepatic porphyria (AHP) a cikin manya.
Bayani: Givlaari sirna ce, wacce ita ce sirna ta biyu da aka amince da ita don tallatawa bayan Onpattro.Hanyar gudanarwa shine allurar subcutaneous.Da miyagun ƙwayoyi ya yi niyya ga mRNA na furotin ALAS1, kuma magani na wata-wata tare da Givlaari na iya rage girman ALAS1 a cikin hanta sosai kuma har abada, ta haka ne rage matakan neurotoxic ALA da PBG zuwa kewayon al'ada, don haka Rage alamun cutar mai haƙuri.Bayanan sun nuna cewa marasa lafiya da aka yi musu magani tare da Givlaari sun sami raguwar 74% a cikin adadin abubuwan da aka kama idan aka kwatanta da ƙungiyar placebo.
(10) Vyondys53
KAMFANI: Sarepta Therapeutics ne ya haɓaka.
Lokacin zuwa kasuwa: FDA ta amince da tallata a watan Disamba 2019.
Alamomi: Don kula da marasa lafiya na DMD tare da dystrophin gene exon 53 splicing maye gurbi.
Bayani: Vyondys 53 maganin antisense oligonucleotide ne, wanda ke yin niyya ga tsarin rarraba dystrophin pre-mRNA.Exon 53 an yanke wani yanki ne, watau baya nan akan balagagge mRNA, kuma an ƙera shi don samar da tsinke amma har yanzu dystrophin mai aiki, don haka inganta ƙarfin motsa jiki a cikin marasa lafiya.
(11) Waylivra
Kamfanin: Ionis Pharmaceuticals ne suka haɓaka da nasa na Akcea Therapeutics.
Lokacin zuwa kasuwa: Hukumar Kula da Magunguna ta Turai (EMA) ta amince da ita don talla a cikin Mayu 2019.
Alamomi: A matsayin maganin adjuvant ban da kula da abinci a cikin manya marasa lafiya tare da familial chylomicronemia syndrome (FCS).
Bayani: Waylivra magani ne na oligonucleotide antisense, wanda shine magani na farko da aka amince don tallatawa a duniya don maganin FCS.
(12) Leqvio
Kamfanin: Novartis ya haɓaka.
Lokacin kasuwa: Tarayyar Turai ta amince da tallata a watan Disamba 2020.
Alamomi: Don lura da manya masu fama da hypercholesterolemia na farko (heterozygous familial da wadanda ba na iyali) ko gauraye dyslipidemia.
Bayanan Bayani: Leqvio sirna ce ta sirna wacce ke nufi PCSK9 mRNA.Ita ce maganin siRNA na farko a duniya don rage cholesterol (LDL-C).Ana gudanar da shi ta hanyar allurar subcutaneous.Magungunan yana rage matakin furotin PCSK9 ta hanyar tsangwama na RNA, don haka rage matakin LDL-C.Bayanai na asibiti sun nuna cewa ga marasa lafiya waɗanda ba za su iya rage matakan LDL-C zuwa matakin da aka yi niyya ba bayan jiyya tare da matsakaicin juzu'i na statins, Leqvio na iya rage LDL-C da kusan 50%.
(13)Oxlumo
Kamfanin: Alnylam Pharmaceuticals ne ya haɓaka.
Lokacin kasuwa: Tarayyar Turai ta amince da tallata a watan Nuwamba 2020.
Alamomi: Don maganin farko na hyperoxaluria nau'in 1 (PH1).
Bayani: Oxlumo magani ne na siRNA wanda ke niyya hydroxyacid oxidase 1 (HAO1) mRNA, kuma hanyar gudanarwa ita ce allurar subcutaneous.An ƙirƙira maganin ta amfani da sabuwar ingantaccen ingantaccen sinadarai na Alnylam, fasahar haɗin gwiwa ta ESC-GalNAc, wacce ke ba da damar sarrafa siRNA a cikin ƙasa tare da juriya da ƙarfi.Magungunan suna lalata ko hana hydroxyacid oxidase 1 (HAO1) mRNA, rage matakin glycolate oxidase a cikin hanta, sa'an nan kuma cinye abin da ake buƙata don samar da oxalate, rage yawan samar da oxalate don sarrafa ci gaban cutar a cikin marasa lafiya da kuma inganta alamun cututtuka.
(14) Viltepso
Kamfanin: NS Pharma, wani reshen Nippon Shinyaku ne ya haɓaka.
Lokacin zuwa kasuwa: FDA ta amince da ita don talla a watan Agusta 2020.
Alamomi: Don maganin Duchenne muscular dystrophy (DMD) tare da exon 53 skipping gene maye gurbi a cikin DMD gene.
Bayani: Viltepso wani maganin antisense oligonucleotide ne wanda zai iya ɗaure zuwa matsayi na exon 53 na pre-mRNA na DMD gene, yana haifar da wani ɓangare na exon 53 da za a cire bayan samuwar mRNA balagagge, ta hanyar gyara wani bangare na gyaran karatun mRNA Akwatin yana taimakawa marasa lafiya su hada wasu nau'o'in marasa lafiya na gajeriyar bayyanar cututtuka na dystrophin na al'ada.
(15) 45
Kamfanin: Sarepta Therapeutics ne ya haɓaka.
Lokacin zuwa kasuwa: FDA ta amince da ita don talla a cikin Fabrairu 2021.
Alamomi: Don maganin Duchenne muscular dystrophy (DMD) tare da exon 45 skipping gene maye gurbi a cikin DMD gene.
Bayani: Amondys 45 maganin antisense oligonucleotide ne, maganin antisense oligonucleotide na iya ɗaure zuwa matsayin exon 45 na pre-mRNA na DMD gene, wanda ya haifar da toshe ɓangaren exon 45 bayan samuwar mRNA Excision balagagge, ta haka ne wani ɓangare na gyara tsarin aikin mRNA na yau da kullun, yana taimakawa wajen daidaita tsarin aikin mRNA. inganta alamun marasa lafiya.
(16) Amvuttra (vutrisiran)
Kamfanin: Alnylam Pharmaceuticals ne ya haɓaka.
Lokaci don kasuwa: FDA ta amince da ita don talla a watan Yuni 2022.
Alamomi: Don lura da transthyretin amyloidosis na gado tare da polyneuropathy (hATTR-PN) a cikin manya.
Jawabai: Amvuttra (Vutrisiran) sirna magani ce da ke nufin transthyretin (ATTR) mRNA, wanda allurar subcutaneous ke gudanarwa.Vutrisiran ya dogara ne akan Alnylam's Enhanced Stability Chemistry (ESC) -GalNAc conjugate isar da dandamali ƙira tare da ƙara ƙarfi da kwanciyar hankali na rayuwa.Yarda da maganin ya dogara ne akan bayanan watanni 9 na nazarin asibiti na Phase III (HELIOS-A), kuma sakamakon gabaɗaya ya nuna cewa maganin ya inganta alamun hatTR-PN, kuma fiye da 50% na yanayin marasa lafiya ya koma baya ko kuma ya daina lalacewa.
4. Sauran magungunan maganin kwayoyin halitta
(1) Rexin-G
Kamfanin: Epeius Biotech ne ya haɓaka.
Lokacin zuwa kasuwa: A cikin 2005, Hukumar Abinci da Magunguna ta Philippine (BFAD) ta amince da ita don talla.
Alamomi: Don maganin ci-gaban ciwon daji masu jurewa chemotherapy.
Bayani: Rexin-G allurar nanoparticle ce da aka ɗora ta akan gado.Yana gabatar da cyclin G1 mutant gene a cikin sel da aka yi niyya ta hanyar retroviral vector don kashe ƙaƙƙarfan ciwace-ciwace.Hanyar gudanarwa shine jiko na cikin jini.A matsayin maganin da aka yi niyya da ƙari wanda ke nema da kuma lalata ƙwayoyin cutar daji na metastatic, yana da wani tasiri mai warkarwa ga marasa lafiya waɗanda suka gaza sauran magungunan kansa, gami da ilimin halitta da aka yi niyya.
(2) Neovasculgen
Kamfanin: Cibiyar Human stem cell ce ta haɓaka.
Lokacin jeri: An amince da shi don jeri a Rasha a ranar 7 ga Disamba, 2011, sannan aka ƙaddamar da shi a Ukraine a cikin 2013.
Alamomi: Don maganin cututtukan cututtuka na jijiyoyin bugun jini, gami da ischemia mai tsanani.
Bayani: Neovasculgen magani ne na kwayoyin halitta bisa DNA plasmids.An gina ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar cuta (VEGF) 165 a kan kashin baya na plasmid kuma an shigar da shi cikin marasa lafiya.
(3) Collategene
Kamfanin: Jami'ar Osaka ne suka haɓaka tare da kamfanonin jari.
Lokaci don kasuwa: Ma'aikatar Lafiya, Ma'aikata da Jindadin Japan ta amince da ita a watan Agusta 2019.
Alamomi: Jiyya na ƙananan ƙarancin ischemia mai mahimmanci.
Bayani: Collategene magani ne na tushen plasmid, magani na farko na jiyya na cikin gida wanda AnGes, kamfanin sarrafa kwayoyin halitta a Japan ya samar.Babban bangaren wannan magani shine plasmid tsirara wanda ke dauke da jerin abubuwan haɓakar hanta na ɗan adam (HGF).Idan an yi amfani da miyagun ƙwayoyi a cikin tsokoki na ƙananan ƙafafu, HGF da aka bayyana zai inganta samuwar sababbin jini a kusa da tasoshin jini da aka rufe.Gwaje-gwaje na asibiti sun tabbatar da tasirinta akan inganta ciwon ciki.
Ta yaya Foregene zai iya taimakawa ci gaban jiyya?
Muna taimakawa adana lokacin nunawa a cikin babban sikeli, a farkon matakin haɓaka magungunan siRNA.
Ziyarci ƙarin cikakkun bayanai:
https://www.foreivd.com/cell-direct-rt-qpcr-kit-direct-rt-qpcr-series/
Lokacin aikawa: Dec-27-2022